New material for solid-state lithium batteries holds promise
The most popular candidates to replace liquid electrolytes include compounds in which lithium ions are surrounded by sulphur or oxygen ions. However, in the journal Advanced Energy Materials, a Swiss-Polish team of scientists has presented a new class of ionic compounds where the charge carriers are lithium ions moving among amine (NH2) and tetrahydroborate (BH4) ions.
The experimental part of the research project was carried out at Empa, the Swiss Federal Laboratories for Materials Science and Technology in Dübendorf, and at the University of Geneva (UG), based on a theory by Prof Zbigniew Lodziana from the Institute of Nuclear Physics of the Polish Academy of Sciences (IFJ PAN) in Cracow.
“We were dealing with lithium amide-borohydride, a substance previously regarded as not being too good an ionic conductor,” said Prof. Lodziana. “This compound is made by milling two constituents in a ratio of 1 to 3 but to date, nobody had ever tested what happens to ionic conductivity when the proportions between these constituents are changed. We were the first to do so and suddenly it turned out that by reducing the number of NH2 groups to a certain limit we could significantly improve the conductivity. It increases so much that it becomes comparable to the conductivity of liquid electrolytes,”
The 50x boost in ionic conductivity of the new material opens up a new, unexplored direction in the search for a candidate for a solid-state electrolyte. “Our lithium amide-borohydride is a representative of a promising new class of solid-state electrolyte materials. However, it will be some time before batteries built on such compounds come into use. For example, there should be no chemical reactions between the electrolyte and the electrodes leading to their degradation,” he said.
The compound was used as an electrolyte in a typical Li4Ti5O12 half-cell which was stable over 400 times. The lithium amide-borohydride exhibited the high ionic conductivity at about 40 °C, and recent experiments have already seen this below room temperature.
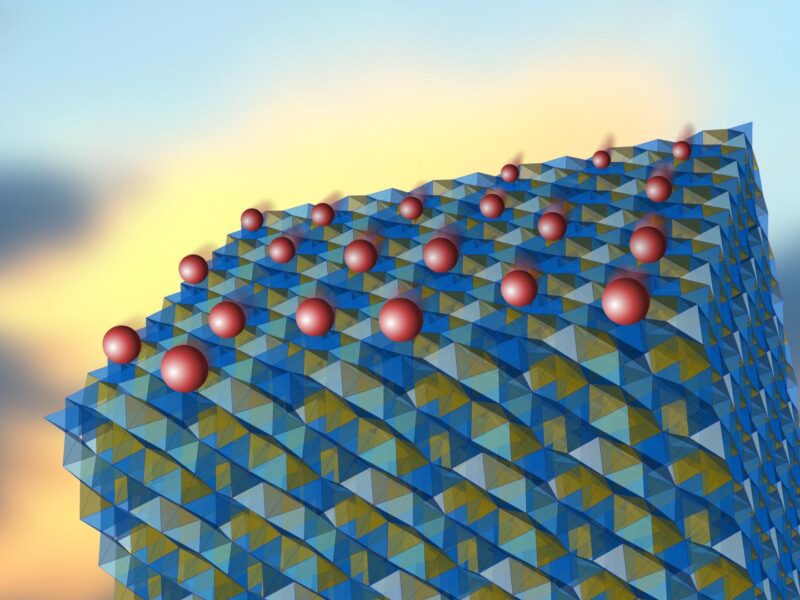
“In the proposed model, the excellent ionic conductivity is a consequence of the specific construction of the crystalline lattice of the tested material. This network in fact consists of two sub-lattices. It turns out that the lithium ions are present here in the elementary cells of only one sub-lattice. However, the diffusion barrier between the sub-lattices is low. Under appropriate conditions, the ions thus travel to the second, empty sub-lattice, where they can move quite freely,” said Prof Lodziana.
Related stories:
SOLID STATE LITHIUM BATTERY HEADS FOR COMMERCIALISATION
SOLID STATE BATTERY MARKET SET TO BOOM
FIRST PRACTICAL SOLID ELECTROLYTE FOR SAFER LITHIUM BATTERIES
BATTERY CHARGES IN SECONDS
SOLID STATE LITHIUM BATTERY TRIPLES CAPACITY
 If you enjoyed this article, you will like the following ones: don't miss them by subscribing to :
eeNews on Google News
If you enjoyed this article, you will like the following ones: don't miss them by subscribing to :
eeNews on Google News