New nanomaterials promise improved harvesting, storage of sunlight
As well as helping improve solar energy technologies, the work may also lead to new applications, such as using sunlight to break down harmful chemicals.
Sunlight drives many chemical processes that would not otherwise occur. For example, carbon dioxide and water do not ordinarily react, but in the process of photosynthesis, plants take these two chemicals and, using sunlight, produce oxygen and sugar.
The efficiency of this reaction is very high, meaning much of the energy from sunlight is transferred to the chemical reaction, but science and engineering have been unable to mimic this process in man-made devices.
One reason is that many molecules that can undergo chemical reactions with light do not efficiently absorb the light themselves. They rely on photocatalysts – materials that absorb light efficiently and then pass the energy on to the molecules to drive reactions.
In the study, researchers investigated an artificial photocatalyst material using nanoparticles and found out how to make it more efficient. This could lead to better solar panels, as the energy from the Sun could be more efficiently harvested. The photocatalyst could also be used to destroy liquid or gas pollutants, such as pesticides in water, by harnessing sunlight to drive reactions that break down the chemicals into less harmful forms.
Lead author Dr Emiliano Cortés from the Department of Physics at Imperial, said: “This finding opens new opportunities for increasing the efficiency of using and storing sunlight in various technologies. By using these materials we can revolutionize our current capabilities for storing and using sunlight with important implications in energy conversion…”
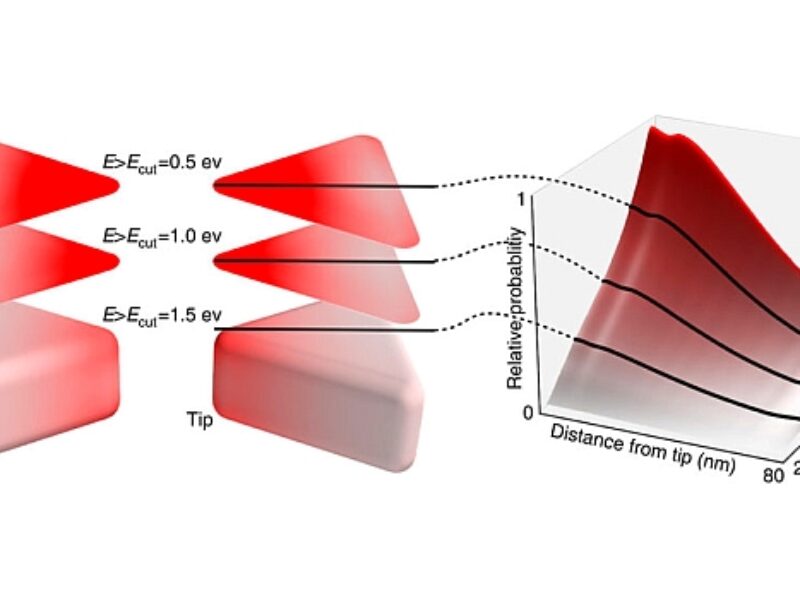
The material that the team investigated comprises metal nanoparticles. The work identified which areas of the nanomaterial would be most suitable for transferring energy to chemical reactions, by tracking the locations of very small gold nanoparticles (used as a markers) on the surface of the silver nanocatalytic material.
Now that they know which regions are responsible for the process of harvesting light and transferring it to chemical reactions, the team hope to be able to engineer the nanomaterial to increase these areas and make it more efficient.
Lead researcher Professor Stefan Maier said: “This is a powerful demonstration of how metallic nanostructures, which we have investigated in my group at Imperial for the last ten years, continue to surprise us in their abilities to control light on the nanoscale.
“The new finding uncovered by Dr Cortés and his collaborators in Germany and the US opens up new possibilities for this field in the areas photocatalysis and nanochemistry.”
For more, see the paper in Nature Communications: ‘Plasmonic hot electron transport drives nano-localized chemistry.’
Related articles:
Can metallic nanoparticles lower the cost of solar cells?
Light harvester reveals quantum physics of photosynthesis
New metamaterial may unlock potential of thermophotovoltaic cells
Hybrid quantum dot material promises improved light harvesting devices
 If you enjoyed this article, you will like the following ones: don't miss them by subscribing to :
eeNews on Google News
If you enjoyed this article, you will like the following ones: don't miss them by subscribing to :
eeNews on Google News