Digital medicines come to cancer patients for first time
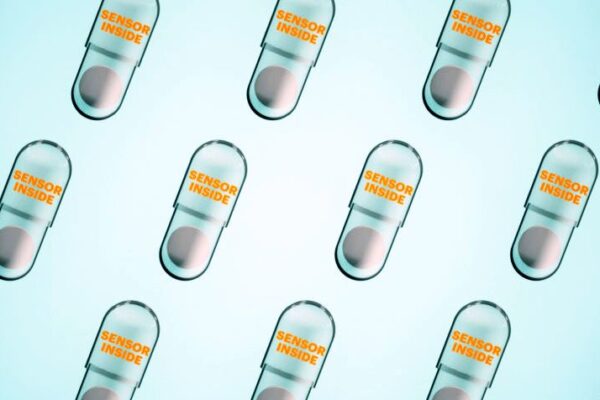
Digital medicines – a category of pharmaceuticals that includes drugs that communicate when they’ve been taken and wearable sensors that capture physiologic response – are currently used in the treatment of conditions like diabetes and hypertension. This new advancement, say the organizations, will help patients complete oral chemotherapy cycles while oncologists gain new insights into their patients’ treatment progress and overall health status.
Proteus developed the care model for oral digital oncology medicines with University of Minnesota Health and Fairview, which is the first health system in the world to prescribe digital capecitabine – a common chemotherapy drug prescribed with the Proteus ingestible sensor. Currently it is being used to help treat stage 3 and 4 colorectal cancer patients.
“Currently, providers make decisions about oral chemotherapy based on patients’ best knowledge of their medication taking,” says Andrew Thompson, CEO and Co-founder of Proteus Digital Health. “For the first time, digital oncology medicines give providers and caregivers new insights and ability to engage with more specific information in the remote care of colorectal cancer patients.”
“Based on our data around the use of digital medicines in other treatment areas,” says Thompson, “we believe this will enable oncology patients to stay on their therapy longer, avoid hospital admissions, and have better response to therapy overall.”
University of Minnesota Physicians oncologist/hematologist Dr. Edward Greeno, who also directs the University of Minnesota Health oncology service line, adds, “Proteus’ digital medicine technology provides a more direct connection to the patient. It creates a way for us to achieve a lot of things that happen when a patient is in the clinic for infusions without them coming in person. Also, we can gain insights about the patient that we can’t learn from an office visit, like how the patient is doing with their treatment regimen while at home, on a daily basis.”
The digital medicines program helps optimize treatment regimens while maintaining a patient’s privacy. The program securely captures, records, and shares information about the time, dose, and type of oral chemotherapy medication taken.
This information – as well as data on rest, activity, and resting heart rate – can be shared with the patient’s consent with their physician, pharmacist, or caretaker. The information can only be seen by the patient and their designated individuals on a secure, mobile-friendly platform developed by Proteus.
Paul Morales, PharmD, BCOP, Fairview Infusion Pharmacy manager at the University of Minnesota Health Clinics and Surgery Center says, “Given the costs, complexity, and toxicity risk for oral chemotherapy, digital oncology medicine is an exciting step forward in cancer care. For pharmacists, it helps us identify patients who might be struggling to take their medication correctly and intervene, for example by giving them a call to explain how to safely move forward if they do miss a dose. For patients, it helps them feel in control as they take a more active role in managing their medication. The results are better outcomes for patients.”
To gather more real-world experience and data from cancer patients using digital medicines, Proteus is launching a digital oral oncolytic medication registry, which will gather data from multiple sites spanning academic medical centers to community practices and urban to rural facilities. Patients from participating sites will be prescribed digital capecitabine to assist them in their treatment. Data collected from the study will be used to share best practices across many sites, enabling richer data and outcome analysis.
“Proteus’ expansion of support for digital medicines into the oncology treatment area is not only important for patients and providers, it will be a game changer for the industry developing therapies intended to one day eradicate cancer,” says Olivia Ware, Proteus’ new Senior Vice President of US Markets and Franchise Development.
Related articles:
FDA approves first ‘digital’ pill
‘Guts Game’ makes play using ingestible sensors
Long-lasting ingestible electronic pill can be wirelessly controlled
‘Smart’ pills, patches can save billions in healthcare costs, says report
Ingestible ‘bacteria on a chip’ can diagnose GI tract disorders
 If you enjoyed this article, you will like the following ones: don't miss them by subscribing to :
eeNews on Google News
If you enjoyed this article, you will like the following ones: don't miss them by subscribing to :
eeNews on Google News